Calcium Citrate
If you are interested on Calcium Citrate, then
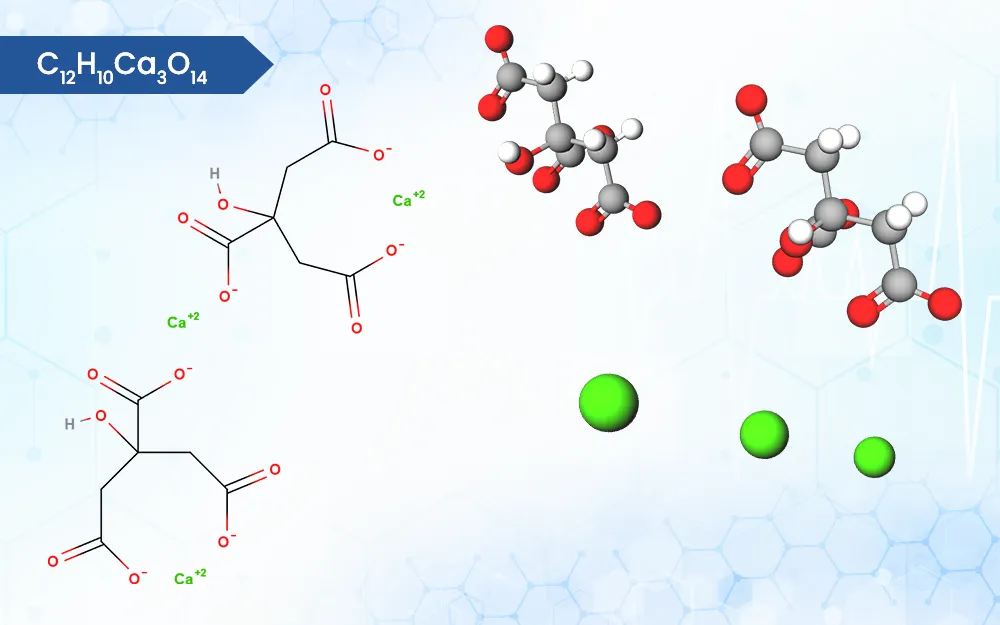
Description of Calcium Citrate
Calcium citrate is the calcium salt of citric acid. It is insoluble in nature. Calcium citrate has 22% of elemental calcium.
Application of Calcium Citrate
- Act as a phosphate binder to reduce phosphate absorption in the digestive tract.
- Used as an active ingredient in antacid medications.
- Used as a buffer agent in pharmaceutical formulations to maintain the desired pH levels
- Chelating agent to complex with metal ions, which can improve the stability and bioavailability of certain drug.
Related Products