Defining API Quality at WBCIL
Welcome to 2026. The nutraceutical landscape has changed dramatically over the last five years. We have moved past the era of blunt instruments—where manufacturers packed enormous dosages into tablets hoping some of it would stick. We are now in the era of precision tools.
Here is the shocking data that forced this industry-wide evolution: pharmacological studies had long highlighted that standard oral administration of vital nutrients—such as curcumin or magnesium oxide—often resulted in absorption rates as dismal as 10% to 20%. For years, consumers were essentially flushing the vast majority of their health investment down the drain.
Today’s educated consumer, and consequently the successful supplement brand, refuses to accept this inefficiency. The market demand has shifted decisively toward advanced delivery systems. This is why understanding the nuances of effective liposomal API application has become the critical success factor for modern formulations.
At West Bengal Chemical Industries Limited (WBCIL), we anticipated this future. We haven’t just adapted to the 2026 standard of quality; we helped define it, positioning ourselves as the best liposomal API manufacturing company in India.
Key Takeaways
- By 2026, the supplement market has decisively shifted from high-dosage guesswork to precision liposomal API application, driven by a consumer demand for products that actually work.
- Successful liposomal API application—ensuring nutrients reach the cells without gastric distress—depends entirely on rigorous manufacturing standards that create stable, nano-sized vesicles.
- As a leading API manufacturing in India, WBCIL sets the global benchmark, offering a validated portfolio of liposomal ingredients that power the most effective product formulations of 2026.

The Mechanism Behind the Application
To appreciate why liposomal API application is transforming the industry, we must first understand the fundamental failure of standard digestion for many nutrients.
The human digestive tract is a hostile environment, designed to break down complex foods with strong acids and enzymes. Many delicate active pharmaceutical ingredients (APIs)—whether vitamins, minerals, or botanical extracts—are destroyed here before they ever reach the small intestine for absorption. Furthermore, once in the intestine, these nutrients often compete for limited transport channels to enter the bloodstream.
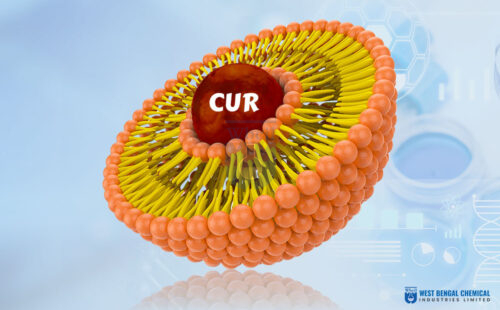
Liposomal technology solves this by utilizing nature’s own design blueprint. A liposome is a microscopic sphere made of a phospholipid bilayer—the exact same healthy fats that compose your own cell membranes.
By encapsulating the active ingredient inside this lipid bubble, we create a microscopic “Trojan Horse.” The digestive system doesn’t recognize it as a raw vitamin to be attacked by acid; it perceives a friendly fat. This shields the nutrient during transit through the stomach. Upon reaching the intestine, the liposome can merge directly with cell walls, delivering its nutrient cargo straight into circulation. This mechanism is the foundation of successful liposomal API application.
Mastering Liposomal API Application in the 2026 Landscape
In the hyper-competitive market of 2026, brands relying on standard active ingredients are struggling to compete with those leveraging advanced liposomal API application. The advantages in final product formulation are simply too significant to ignore.
1. Enabling Lower-Dose, High-Efficacy Application
The most significant impact of effective liposomal API application is radically enhanced bioavailability. By bypassing standard digestive bottlenecks, liposomes ensure a much higher percentage of the nutrient reaches systemic circulation. This allows formulators to apply lower, more economical dosages while achieving superior biological effects compared to massive doses of conventional powders.
2. Improving Compliance in Sensitive Applications
We are all familiar with the consumer complaints: iron supplements causing severe stomach cramping, or high-dose Vitamin C leading to acidity. Because the active ingredient is shielded within a fat layer, it doesn’t make direct, harsh contact with the stomach lining. A primary advantage of liposomal API application is the ability to deliver potent nutrients gently, ensuring consumers actually stick to their regimen.
3. Expanding Application to Sensitive Ingredients
Many valuable ingredients, such as glutathione or certain antioxidant enzymes, oxidize rapidly when exposed to air or stomach acid, rendering them useless in standard forms. Successful liposomal API application provides a protective barrier, ensuring these fragile ingredients remain stable on the shelf and active in the body.
Defining Quality in Liposomal API Application: Why Not All Are Equal
This is the 2026 reality check. As liposomal technology gained popularity, the market was flooded with “liposomal” products that were, in reality, mere emulsions—simple, unstable mixtures of oil, water, and vitamins lacking real liposomal structure.
To achieve genuine results in liposomal API application, you need rigorous science, not just marketing buzzwords. Quality in this sector is defined by three non-negotiable factors:
1. Particle Size is Crucial for Application
For a liposome to effectively bypass standard digestion, it needs to be nano-sized, typically under 200 nanometers. If the particles are too large, the body treats them like standard dietary fat, negating the unique advantages of the application. Achieving this uniform size requires sophisticated high-pressure homogenization, not simple blending.
2. The Source of Phospholipids
The quality of the “bubble” dictates the quality of the final product. At WBCIL, a leading API manufacturing in India, we utilize high-grade phosphatidylcholine, often sourced from non-GMO sunflowers. Inferior lipids lead to unstable liposomes that leak their nutrient payload before consumption.
3. Stability Ensures Successful Application
A liposome must remain a liposome until ingestion. Many inferior products separate over time. True quality means rigorous stability testing to ensure the structure holds up, guaranteeing consistent liposomal API application throughout the product’s shelf life.
WBCIL: The Indian Benchmark for Global Quality
India has emerged as a global pharmacy hub, and in 2026, it is also the center for advanced nutraceutical technology. WBCIL is at the forefront of this evolution, recognized widely as the best liposomal API manufacturing company in India.
We don’t just sell ingredients; we provide validated science that enables superior product applications. Our manufacturing facility employs state-of-the-art homogenization equipment and rigorous quality control protocols. When partners choose WBCIL, they aren’t just buying an API; they are securing the success of their final liposomal API application.
The WBCIL Portfolio: Powering 2026 Product Applications
Our commitment to quality manufacturing translates directly into our product offerings. We deliver validated ingredients ready for high-performance liposomal API application across a broad spectrum of health categories.
Current Liposomal APIs for Immediate Application
We currently manufacture a robust range of stable, high-bioavailability liposomal APIs:
- Liposomal Iron (Ferrous Bisglycinate / Ferrous Ascorbate):Application: The definitive solution for anemia formulations. It offers high absorption without the notorious side effects of constipation or metallic taste, revolutionizing iron therapy compliance.
- Liposomal Vitamin C (Ascorbic Acid / Sodium Ascorbate):Application: Ideal for high-dose immune support and collagen-boosting beauty-from-within products, without causing gastric acidity or diarrhea.
- Liposomal Magnesium (Bisglycinate / Citrate):Application: Perfect for muscle recovery, sleep aid, and stress relief formulations, delivering magnesium directly to cells without the dreaded laxative effect of standard salts.
- Liposomal Curcumin:Application: Unlocks the potent anti-inflammatory potential of turmeric for joint health and cognitive support products by overcoming standard curcumin’s notoriously poor absorption.
- Liposomal Glutathione:Application: Essential for premium detoxification, skin brightening, and immune defense formulations, protecting the “master antioxidant” from destruction by stomach acid.
- Liposomal Vitamin D3 + K2:Application: A synergistic blend for advanced bone and heart health products, ensuring efficient absorption even in individuals with compromised fat digestion.
- Liposomal Zinc:Application: Provides gentle yet potent immune support, avoiding the nausea often associated with standard zinc supplements taken on an empty stomach.
- Liposomal CoQ10 (Ubiquinone): For advanced cellular energy and cardiovascular health formulations, overcoming the absorption challenges of this large molecule.
Upcoming APIs Expanding Liposomal Application
The market of 2026 never stands still, and neither do we. Our R&D pipeline is finalizing the next wave of ingredients optimized for liposomal API applications
Conclusion
The nutraceutical market of 2026 is demanding, transparent, and science-driven. The era of formulating with poorly absorbed ingredients is over. Liposomal technology has proven itself to be the superior method for delivering essential nutrients.
However, realizing the full potential of liposomal API application requires precision engineering and unwavering quality standards. As a leading API manufacturing in India, WBCIL is proud to set that benchmark, providing the high-performance ingredients that power the next generation of effective health products.
In application, a standard API (like generic vitamin powder) is exposed directly to stomach acid and relies on inefficient transport systems. A liposomal API is encapsulated, allowing it to bypass stomach degradation and be absorbed directly by cells, leading to significantly higher efficacy in the final product.
True liposomal technology is inherently liquid-based, involving lipids suspended in water. We specialize in high-stability liquid liposomal APIs ready for easy application into syrups, drops, or soft gels.
This is the defining question of quality. We use advanced analytical techniques, such as Dynamic Light Scattering (DLS), to verify particle size distribution, ensuring we have created true, nano-sized liposomes rather than large, unstable fat globules.
In 2026, we combine world-class pharmaceutical engineering expertise with cost-effective manufacturing. Our reputation is built on rigorous scientific validation, stability testing, and a commitment to producing true nano-liposomes, not just marketing hype.
The technology is incredibly versatile. It is excellent for water-soluble ingredients (like Vitamin C, trapped in the core) and fat-soluble ingredients (like Curcumin, embedded in the lipid layer). Our portfolio demonstrates this versatility across varied applications.