WBCIL’s Pharmaceutical Patents: A Commitment to Progress
West Bengal Chemical Industries Limited (WBCIL) holds 9 pharmaceutical patents, evidencing leadership in healthcare innovation. These patents cover a range of molecules, including Ferric Carboxymaltose, highlighting WBCIL’s commitment to advancing medical science and enhancing health and well-being globally. Our patented developments showcase breakthroughs in reducing side effects and achieving cost-effectiveness, underscoring our dedication to progress and excellence in the pharmaceutical industry.
WBCIL Achieves Global Recognition with Certificate of Pharmaceutical Product
The Certificate of Pharmaceutical Product (CPP of West Bengal Chemical Industries Limited (WBCIL) signifies our compliance with international regulatory standards for exporting pharmaceutical products. Such certificates confirm that our products are approved for marketing within nationally and internationally and meet the necessary quality, safety, and efficacy standards, allowing them to be considered for importation by other countries. This accreditation is crucial for WBCIL’s global business operations, ensuring our APIs are recognized on an international platform.
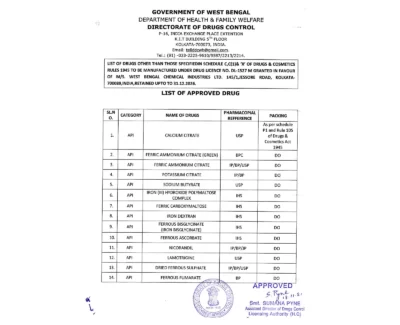
West Bengal Chemical Industries Limited is a proud patent certificate holder of 9 molecules:
- Ferric Carboxymaltose
- Iron-Isomaltoside
- Iron (III) Coordination Complex
- Ferric Derisomaltose
- Ferric Citrate
- Sucroferric Oxyhydroxide
- Enclomiphene Citrate
- Improved Ferric Carboxymaltose with Less Side-Effects Obtained Cost Effectively
- Ferric Maltol: Preparation of Novel Polymorphic Form ‘s’ of Stabilised Ferric Maltol and Scalable Process
These certificates serve as a testament to our commitment to pushing the boundaries of science and improving the world’s health and well-being. Proudly display these achievements as a symbol of our dedication to innovation and progress.
Here are some major certification details:
West Bengal Chemical Industries Limited showcases a strong commitment to quality and regulatory compliance through its acquisition of various certificates:
- Pharmaceutical Export Promotion Council of India (Pharmexcil): Demonstrates WBCIL’s engagement in global pharmaceutical export initiatives.
- Quality Management System (QMS): Indicates adherence to international standards for quality assurance in manufacturing processes.
- Importer-Exporter Code (IEC): A key certification for companies involved in international trade, reflecting WBCIL’s capability to export and import goods.
- Hazard Analysis Critical Control Point (HACCP): This certification underscores WBCIL’s commitment to food safety and quality control in its operations.
- Food Safety and Standards Authority of India (FSSAI): Ensures compliance with food safety regulations, critical for nutraceutical products.
These certifications highlight our dedication over 60 years and maintaining high standards in product quality, safety, and regulatory compliance, facilitating reputation as a trusted name in the pharmaceutical and nutraceutical industry.