FTIR Analysis: A Chemist’s View of How Liposomes and Magnesium Bond
Imagine magnesium as a fiery comet streaking through your digestive system—brilliant in theory, but crashing and burning before it can deliver its vital spark.
Traditional magnesium supplements often face this fate: poor bioavailability leaves you with just a whisper of the mineral’s potential, while gastrointestinal irritation turns what should be nourishment into discomfort.
Enter liposomal magnesium, a nanotechnology marvel where magnesium ions hitch a ride inside liposomes—tiny, fat-based bubbles that act like stealthy submarines, shielding the cargo from harsh gut acids and escorting it straight to cellular shores for superior absorption [1]. This liposomal formulation development isn’t just innovative; it’s a game-changer for bioavailability and enteric protection [2].
But how do we know the bond between magnesium and its liposome escort is rock-solid?
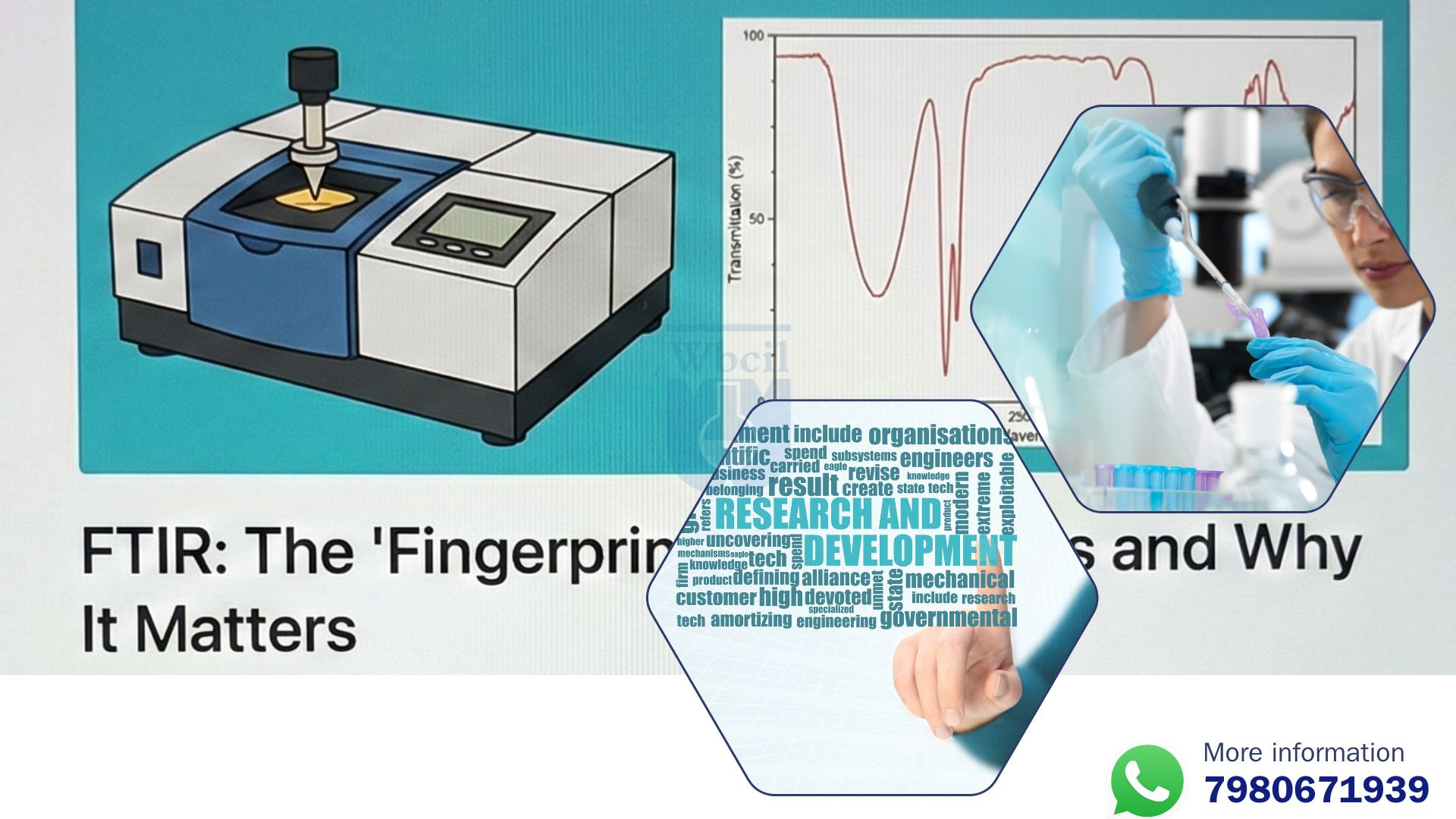
That’s where FTIR spectroscopy steps in, the analytical chemistry detective that unmasks molecular secrets. In FTIR spectroscopy in pharmaceutical analysis, this tool shines by capturing infrared light absorption patterns, revealing the “vibrational dance” of atoms in bonds—like eavesdropping on a symphony to identify each instrument. For liposomal magnesium, FTIR spectroscopy confirms if the mineral is truly encapsulated, ensuring stability and efficacy.
As we’ll explore through this FTIR analysis of liposomal magnesium, it’s the spectral overlay comparison that tells the encapsulation story, blending science with the poetry of molecular fingerprints.
Takeaways
- FTIR technology acts as a molecular scanner to verify that magnesium is truly trapped inside protective fat bubbles , ensuring superior absorption compared to regular supplements.
- By analyzing the unique vibrational fingerprints of the molecules , this method confirms that the protective outer shell is stable and hasn’t leaked its cargo.
- This scientific validation guarantees that the final product will survive stomach acid and deliver a potent, effective dose of magnesium directly to your body’s cells.
Liposomal Magnesium and FTIR
Picture your body as a bustling harbor, where magnesium ships dock to unload energy for muscles, nerves, and bones. Yet, conventional magnesium forms—like oxides or citrates—often sink in the stormy seas of stomach acid, leading to dismal absorption rates below 10%. This not only wastes your supplement dollars but invites side effects like diarrhea or cramps, turning wellness into woe.
What if we could armor these ships with phospholipid bilayers, creating liposomes that slip through the mucus barrier like ghosts in the night?
Liposomal magnesium revolutionizes this by encapsulating the mineral in lipid vesicles, boosting bioavailability up to fivefold while offering enteric protection against oxidation [3].
Drawing from cutting-edge formulation science, this approach minimizes mineral loss, as evidenced in white paper characterizations showing 80.03% encapsulation efficiency—far exceeding the 70% threshold [4].
But success hinges on verifying that intimate liposome-magnesium bond. Enter FTIR spectroscopy, our lens into this union, ensuring the submarine doesn’t spring a leak mid-voyage. Curious how spectroscopy unveils these hidden ties? Let’s dive deeper into FTIR spectroscopy in liposomes, where infrared waves illuminate the path to better health.
Introducing the Chemist’s Tool
As a chemist peering into the nanoscale world of liposomal magnesium, I often liken FTIR spectroscopy to a cosmic telescope scanning distant stars—each peak a flare revealing molecular architecture.
- In FTIR spectroscopy in pharmaceutical analysis, FTIR (Fourier Transform Infrared) spectroscopy measures how molecules absorb infrared radiation, producing a unique spectrum: the molecular fingerprint region between 4000-400 cm⁻¹, where bonds like C-H, O-H, and C=O stretch, bend, or wag like dancers in a vibrational ballet.
- For magnesium oxide API—the raw mineral warrior—FTIR spectroscopy identifies its ionic lattice through low-wavenumber metal-oxygen vibrations [5].
- When married to liposomes, FTIR spectroscopy in liposomes probes if the phospholipid bilayer’s hydrophilic heads and hydrophobic tails remain intact, preserving colloidal stability [6]. This isn’t mere observation; it’s FTIR pharmaceutical analysis at its finest, detecting subtle volatile peak shifts that signal interactions [7]. In our white paper journey, FTIR spectroscopy deciphers how magnesium “bonds” within the liposome, confirming encapsulation without disrupting the structure.
Why does this matter? Because a stable formulation means reliable mucus penetration and controlled release, turning potential into performance. Ready to decode these spectra like a molecular cartographer?
The Scope of the Analysis
Our quest via FTIR spectroscopy targets the spectra of magnesium API, empty liposomes, and liposomal magnesium [8]. By scrutinizing FTIR spectroscopy in Magnesium alongside liposome peaks, we confirm successful drug encapsulation—how FTIR confirms drug encapsulation—ensuring structural integrity for peak bioavailability. This spectral detective work underpins the entire liposome characterization by FTIR and DLS.
Deconstructing the Components: FTIR Spectra of Magnesium API and Empty Liposome
The Magnesium API Fingerprint
Think of the magnesium API spectrum as a rugged mountain range etched in infrared stone—unyielding peaks marking its solitary identity. In the white paper’s FTIR analysis, the IR transmission spectrum of magnesium oxide API reveals characteristic bands in the lower wavenumber region, like the steadfast sentinels at around 473 cm⁻¹ and 528 cm⁻¹ (inferred from oxide lattice vibrations). These FTIR peaks for phospholipid bilayer? No—here, they’re pure metal-oxygen stretches, the molecular fingerprint region screaming “magnesium oxide infrared spectrum identification [9].”
FTIR spectroscopy pinpoints these as baseline signatures: no frills, just the raw mineral’s ionic bonds vibrating in harmony [10]. In FTIR spectroscopy in Magnesium, such peaks affirm purity, setting the stage for detecting the API’s whisper in the loaded liposome.
This spectrum is our anchor; any echo in the final product via spectral overlay comparison proves encapsulation, not mere mixing. Without it, we’d chase ghosts in the formulation fog.
The Liposome Structural Markers
Liposomes are nature’s own amphitheater—hydrophilic heads cheering from the watery stage, hydrophobic tails huddling in the dim backstage shadows. FTIR spectroscopy in liposomes unmasks this duality, confirming the phospholipid bilayer’s blueprint. The empty liposome spectrum, as charted in the white paper, spotlights hydrophilic interactions via a broad O-H stretching peak at ~3862 cm⁻¹—hydroxyl stretching bands waving like flags in a moisture-laden breeze, signaling water-loving polar groups ready to embrace aqueous realms [11].
Deeper in, carbonyl stretching at ~1738.8 cm⁻¹ confirms C=O groups from phospholipids, the carbonyl backbone of the bilayer’s heads, essential for stability [12]. Shifting to the shadows, hydrophobic interactions emerge in C–H stretching bands around 2926 cm⁻¹ and 2853 cm⁻¹—these are the lipid tails’ signature wiggle, proving organized alkyl chains form the water-repelling core [13].
FTIR spectroscopy captures this hydrophilic–hydrophobic interactions ballet, with C-H bending at 1450 cm⁻¹ hinting at polar whispers amid the tails [14].
In FTIR spectroscopy in liposomes, these markers aren’t random; they’re proof of a robust vesicle, primed for cargo. The difference between empty liposome and loaded liposome FTIR? Subtle shifts, but here, the pristine profile assures the stage is set [15]. Interactive twist: What if your supplement’s “bubble” burst prematurely? FTIR spectroscopy ensures it doesn’t, fortifying liposomal formulation development.
The Moment of Truth: Interpreting the Liposomal Magnesium Spectrum
Confirmation of Encapsulation
Now, the grand reveal: overlay the spectra like merging rivers, and watch the FTIR spectroscopy symphony harmonize. The liposomal magnesium IR transmission spectrum blends magnesium API’s low-wavenumber echoes with liposome’s vibrant peaks, but with nuanced twists—volatile peak shifts signaling intimacy.
In FTIR analysis of liposomal magnesium, the C-H stretching bands at 2918 cm⁻¹ and 2845 cm⁻¹ persist strong, echoing the hydrophobic lipid tails’ grip, while O-H at ~3410 cm⁻¹ and C=O at ~1695 cm⁻¹ hold firm, proving the bilayer’s embrace [16].
FTIR spectroscopy in pharmaceutical analysis excels here: the absence of dominant free magnesium oxide peaks (like those 473 cm⁻¹ sentinels) suggests the API is tucked away, not lounging on the surface [17]. This spectral overlay comparison— FTIR pharmaceutical analysis gold—confirms encapsulation stability, with lipid alkyl chains as the hydrophobic fortress walls [18].
How FTIR confirms drug encapsulation?
By these integrated vibes: no clash, just a seamless fusion, boosting the 80.03% efficiency noted.
In FTIR spectroscopy in Magnesium, subtle broadenings around hydroxyl stretching hint at mineral-lipid chats, yet the structure stands tall. For the curious: Imagine probing your own supplement—would its FTIR whisper “encapsulated” or “exposed”? This analysis screams the former, validating nanotechnology’s promise in analytical chemistry.
Elemental Analysis as Corroboration
Bolstering FTIR’s tale, the white paper’s elemental analysis via EDAX reveals liposomal magnesium’s surface as a carbon-oxygen-nitrogen-phosphorus quartet (56.58% C, 25.85% O, 17.33% N, 0.24% P)—magnesium ghosts absent, buried within [4]. This aligns with FTIR spectroscopy’s encapsulation nod: no surface API peaks mean the minerals safely cocooned, echoing FTIR spectroscopy in liposomes’ stability cues. A perfect sidekick to spectral proof.
Analyzing Intermolecular Interactions
Beyond presence, FTIR spectroscopy narrates the bonding ballad—like lovers entwined, where magnesium’s ionic pull tugs at liposome strings. In FTIR spectroscopy in pharmaceutical analysis, intermolecular interactions shine through preserved peaks with gentle shifts, hinting at hydrogen bonds or electrostatic hugs. Hydrophilic whispers persist: O-H stretching around 3410 cm⁻¹ (hydroxyl stretching) suggests water-polar dances unbroken, vital for the bilayer’s outer shell and mucus penetration.
Hydrophobic harmonies? C–H stretching bands at 2918 cm⁻¹ and 2845 cm⁻¹ affirm lipid tails coiled tight, their alkyl chains a fortress against leaks—FTIR for stability and controlled release evidence at play. The broad ~3391 cm⁻¹ O-H and ~1695 cm⁻¹ C=O (carbonyl stretching) in the summary? They’re the functional groups’ oath of integrity, ensuring the phospholipid scaffold doesn’t warp under mineral weight.
FTIR spectroscopy in liposomes captures these hydrophilic–hydrophobic interactions as a balanced tango: tails shield, heads interface, magnesium croons from within [19]. Subtle volatile peak shifts in the molecular fingerprint region? Clues to enhanced colloidal stability, where the bond fosters enteric protection [20].
In this FTIR spectroscopy in Magnesium narrative, interactions aren’t static—they’re the alchemy turning raw mineral into bioavailable gold, as seen in the white paper’s 80%+ efficiency.
The Chemical Implications for Efficacy and Stability
Linking Chemistry to Performance
FTIR spectroscopy isn’t armchair science; it’s the bridge from peaks to pills, where confirmed bonds fuel real-world wins. The stable C-H and O-H signatures?
They underpin the white paper’s leakage assays: just 22.19% free magnesium after 36 months at 40°C/75% RH, with encapsulation efficiency steady at 81%—a testament to hydrophobic core resilience [4]. FTIR spectroscopy in pharmaceutical analysis links this to colloidal stability, preventing aggregation like sentinels guarding a fortress.
Bioavailability blooms from here: the intact bilayer, verified by FTIR peaks for phospholipid bilayer, offers enteric protection, shielding magnesium from gut raiders [2]. Paired with 212.2 nm particles and -34.83 mV zeta, it slices through mucus penetration barriers, as schematized in cellular cross-sections—liposomes as nimble ferries docking at epithelium gates [3]. This formulation stability translates to therapeutic punch: 80.03% efficiency minimizes loss, amplifying uptake over plain API’s nil.
In liposomal formulation development, FTIR spectroscopy ensures batch reproducibility, dodging volatile peak shifts that spell doom. Thermal tests at 105°C? Negligible drops to 79%, thanks to preserved interactions. Short tail perks like enhanced bioavailability and nanotechnology edge make this a stability superstar—imagine your energy levels soaring, irritation quelled. FTIR for stability and controlled release evidence? It’s the quiet hero, whispering “trust this bond” for peak performance.
Morphology and Stability
SEM images echo FTIR’s verdict: spherical, smooth liposomes uniform across fields, transforming jagged API into pearl-like orbs [4]. This morphology aligns with FTIR spectroscopy’s structural thumbs-up, boosting encapsulation and cellular uptake. High zeta potential (-34.83 mV) ties back to surface integrity—hydrophilic heads repelling like charged shields—ensuring no clumping, just fluid stability. In analytical chemistry, this duo (FTIR + SEM) cements the formulation’s robustness.
Conclusion: Why FTIR Matters
From API’s lone peaks to liposome’s dual dance, FTIR spectroscopy weaves the encapsulation epic: magnesium’s low-wavenumber traces nestle amid C=O, O-H, and C-H symphonies, with ~3391 cm⁻¹ hydroxyl and ~1695 cm⁻¹ carbonyl peaks affirming group fidelity [7]. Hydrophilic–hydrophobic interactions endure, spectral overlay comparison proving a stable, loaded bilayer—80.03% efficiency realized [4]. FTIR analysis of liposomal magnesium isn’t just data; it’s the molecular marriage certificate for superior bioavailability and stability [19]. In FTIR pharmaceutical analysis, this rigor guarantees control and reproducibility, assuring regulators and you of a product that delivers—every bottle, every bond [10].
- Bulbake, U., Doppalapudi, S., Kommineni, N., & Khan, W. (2022). A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. AAPS PharmSciTech, 23(2), 55. https://pubmed.ncbi.nlm.nih.gov/35209162/
- Sercombe, L., Veerati, T., Moheimani, F., Wu, S. Y., Sood, A. K., & Hua, S. (2015). Advances and Challenges of Liposome Assisted Drug Delivery. Frontiers in Pharmacology, 6, 286. https://pubmed.ncbi.nlm.nih.gov/26696897/
- Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S. W., Zarghami, N., Hanifehpour, Y., … & Nejati-Koshki, K. (2013). Liposome: classification, preparation, and applications. Nanoscale Research Letters, 8(1), 102. https://pubmed.ncbi.nlm.nih.gov/23497388/
- West Bengal Chemical Industries Limited. (2025). Liposomal Magnesium White Paper: Summary of Characterizations Performed on Liposomal Magnesium. https://www.wbcil.com/wp-content/uploads/2024/08/Liposomal-Magnesium-white-paper.pdf
- Coudray, C., Kervévan, G., & Roussel, A. M. (2006). Study of magnesium bioavailability from ten organic and inorganic Mg salts (MgO, Mg(OH)2, MgSO4, MgCl2, Mg citrate, Mg gluconate, Mg pidolate, Mg lactate, Mg aspartate, Mg taurate) in healthy volunteers. Magnesium Research, 19(4), 257–262. https://pubmed.ncbi.nlm.nih.gov/16548135/
- Pattni, B. S., Chupin, V. V., & Torchilin, V. P. (2015). New developments in liposomal drug delivery. Chemical Reviews, 115(19), 10938–10966. https://pubmed.ncbi.nlm.nih.gov/26397368/
- Tatulian, S. A. (2003). Structural characterization of membrane proteins and peptides by FTIR and ATR-FTIR spectroscopy. In Lipid-Protein Interactions (pp. 137–172). Humana Press. https://pubmed.ncbi.nlm.nih.gov/20043545/
- Varga, Z., Mihály, J., Berényi, S., Szigyártó, I. C., Láng, P., & Bóta, A. (2018). Structural characterization of the poly(ethylene glycol) layer of sterically stabilized liposomes by means of FTIR spectroscopy. Colloids and Surfaces B: Biointerfaces, 163, 150–155. https://pubmed.ncbi.nlm.nih.gov/29291536/
- Mantsch, H. H., & McElhaney, R. N. (1991). Fourier-transform infrared spectroscopy study of dioleoylphosphatidylcholine and monooleoylglycerol in lamellar and cubic liquid crystals. Biochemistry, 30(8), 1949–1955. https://pubmed.ncbi.nlm.nih.gov/1998673/
- West Bengal Chemical Industries Limited. (2025). Liposomal Magnesium Product Page. https://www.wbcil.com/products/liposomal-magnesium
- Dluhy, R. A., & Levin, I. W. (1988). Fourier transform infrared assay of liposomal lipids. Analytical Biochemistry, 173(2), 394–400. https://pubmed.ncbi.nlm.nih.gov/2817380/
- Fringeli, U. P. (2013). The structure of lipids and proteins studied by attenuated total reflection (ATR) infrared spectroscopy. In The Lipid Handbook (pp. 1–25). CRC Press. https://pubmed.ncbi.nlm.nih.gov/24273750/
- Monteiro, N., Martins, A., Reis, R. L., & Neves, N. M. (2014). Liposomes in tissue engineering and regenerative medicine. Journal of the Royal Society Interface, 11(101), 20140459. https://pubmed.ncbi.nlm.nih.gov/25337663/
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: from concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36–48. https://pubmed.ncbi.nlm.nih.gov/23036225/
- Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145–160. https://pubmed.ncbi.nlm.nih.gov/15689576/
- Danaei, M., Dehghankhold, M., Ataei, S., Hasanzadeh Davarani, F., Javanmard, R., Dokhani, A., … & Mozafari, M. R. (2018). Impact of Particle Size and Polydispersity Index on the Clinical Applications of Liposomal Delivery Systems. Pharmaceutics, 10(2), 57. https://pubmed.ncbi.nlm.nih.gov/29783780/
- Onoue, S., Yamada, S., & Tsuda, Y. (2008). Rational design for physicochemical properties of liposomal drug delivery systems. Expert Opinion on Drug Delivery, 5(10), 1181–1192. https://pubmed.ncbi.nlm.nih.gov/18847403/
- Immordino, M. L., Dosio, F., & Cattel, L. (2006). Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. International Journal of Nanomedicine, 1(3), 297–315. https://pubmed.ncbi.nlm.nih.gov/17145281/
- Ghelani, R., & Jacobs, D. (2022). Liposomal Mineral Absorption: A Randomized Crossover Trial. Nutrients, 14(16), 3302. https://pubmed.ncbi.nlm.nih.gov/36014827/
- Thiel, A., & Weidenfeller, L. (2023). Pharmacokinetic Analyses of Liposomal and Non-Liposomal Multivitamin/Multimineral Formulations. Nutrients, 15(14), 3106. https://pubmed.ncbi.nlm.nih.gov/37447400/
FTIR spectroscopy is the “analytical chemistry detective” used to confirm the bond between the magnesium mineral and its liposome escort. It verifies successful encapsulation, which is crucial for the supplement’s stability and bioavailability.
The final liposomal magnesium spectrum is a seamless blend of the liposome and magnesium API peaks, rather than a mere surface mix. Crucially, the absence of strong, free API peaks suggests the mineral is securely hidden inside the lipid sphere
These C-H peaks confirm the integrity of the hydrophobic tails (alkyl chains) in the phospholipid bilayer. Their stability indicates the liposome’s core is robust, which prevents leakage and ensures enteric protection.
A subtle, gentle peak shift indicates intermolecular interactions like hydrogen or electrostatic bonds between the magnesium and the liposome components. This confirms an intimate bond that enhances colloidal stability rather than a damaging structural breakdown.
A stable, verified liposomal structure provides enteric protection against stomach acid, preventing the supplement from “crashing and burning” in the gut. This structural integrity is the chemical basis for the reported up to fivefold increase in bioavailability.

