Copper Propionate
-
Product Name:
Copper Propionate
-
Molecular Formula:
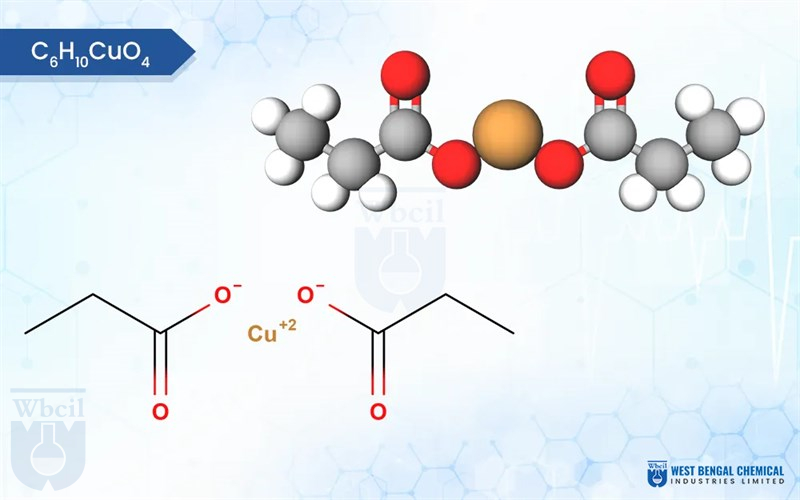
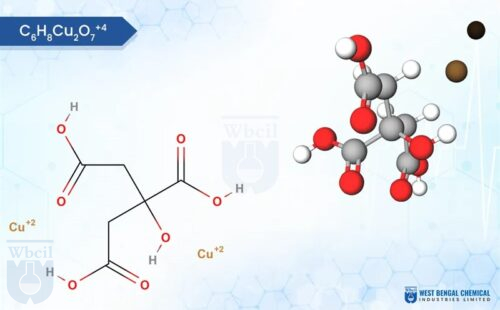
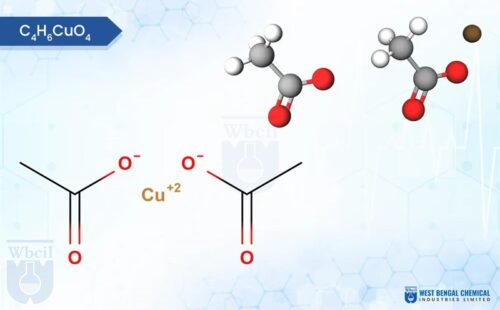
C6H10CuO4
-
Molecular Weight:
209.69 g/mol
-
CAS No.:
3112-74-1
-
HSN Code:
29155000
-
CID Code:
13211378
-
Shelf Life:
3 years - 20°C powder
-
ChemSpider ID
13733748
-
UNII No.
Q5OQP4HQ2B
- USP
- IUPAC Names
- Synonyms
- MSDS
- Specification
USP of Copper Propionate
Copper propionate is used as a fungicide in agriculture to control various plant diseases. It is effective against fungal pathogens that affect crops.
IUPAC Names of Copper Propionate
Calcium dipropanoate
Synonyms of Copper Propionate
- Cupric propionate
- 3112-74-1
- copper propionate
- copper;propanoate
- Copper(II) propionate
- Copper dipropionate
- Propanoic acid, copper(2+) salt
- Propionic acid, copper(2+) salt
- Copper Dipropanoate
- Copper(ii)propionate
- Copper(2+) dipropanoate
MSDS of Copper Propionate
Download MSDS PDFChemical Product and Company Identification
- Product name: Copper Propionate
- Product Code: CPR3032
First Aid Measures
- Eye Contact: Flush eyes with large amounts of water for fifteen minutes. Separate eyelids with fingers. If irritation persists, seek medical attention.
- Skin Contact: Wash skin with soap and water. If irritation persists, seek medical attention.
- Ingestion: Do not induce vomiting. Seek medical attention.
- Inhalation: Move to a fresh air environment. Contact a physician if breathing becomes difficult.
Fire-Fighting Measures
- Flash Point: Not Available
- Explosion Limits:
Lower: Not Available
Upper: Not Available - Auto Ignition Temperature: Not Available
- Extinguishing Media: Carbon dioxide, dry chemical powder, alcohol- resistant foam or water spray.
- Protective Equipment: Wear self-contained respirator and fully protective impervious suit.
- Specific Hazards: May emit hazardous fumes under fire conditions
Accidental Release Measures
- Personal Protection: Wear a self-contained breathing apparatus, rubber boots and gloves, and disposable coveralls. Dispose of coveralls after use. Keep unprotected persons away.
- Environmental Protection: Keep spills out of sewers and bodies of water. Dike and contain the spill with inert material. Absorb on sand, vermiculite or diatomite. Transfer material to a container for disposal or recovery. Ventilate area and wash spill site after material pickup is complete.
Handling and Storage
- Handling: Avoid breathing dust, vapor, mist or gas. Avoid contact with skin and eyes. Avoid prolonged or repeated exposure. Use only in a chemical fume hood. Open and handle container with care. Keep ignition sources away.
- Storage: Store in a tightly closed container in a dry, well- Ventilated place.
- Sensitivities: Not Available
- Storage Temperature: 15 – 30 ºC
References
- https://whmis.org/sds/
- https://www.osha.gov/sites/default/files/publications/OSHA3514.pdf
- https://reachonline.eu/reach/en/annex-ii.html
- https://www.cdc.gov/niosh/npg/
- https://pubchem.ncbi.nlm.nih.gov/compound/Cupric-propionate#section=Other-Identifiers
- https://www.chemspider.com/Chemical-Structure.13733748.html
- https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5775190.htm
If you are interested on Copper Propionate, then
Description of Copper Propionate
Copper propionate is a chemical compound that contains copper and propionic acid. Copper propionate is sparingly soluble in water, meaning it dissolves only to a limited extent. It is more soluble in organic solvents like ethanol and acetone. Copper propionate is usually found as a blue or greenish-blue powder or crystalline solid. Its color is attributed to the presence of copper ions.
If you are interested on Copper Propionate, then
Application of Copper Propionate
- Copper propionate has been shown to have antimicrobial activity against bacteria, viruses, and fungi, making it a promising candidate for use in wound dressings, textiles, and other medical applications.
- It is used as the reagent in the preparation of superconductors & semiconductors.
- Copper propionate has been shown to have anti-inflammatory properties.
Related Products
If you are interested on Copper Propionate, then
Related Blogs of Copper Propionate

Imagine a vibrant mosaic, where each tile, meticulously chosen and placed, contributes to a breath-taking masterpiece. In the intricate mosaic…...

Aging is more than just the appearance of wrinkles and gray hair—it’s a multidimensional process that impacts our physical health,…...

In today’s world, our bodies constantly encounter toxins—whether from environmental pollution, processed foods, stress, or negative emotions. Over time, these…...

In the intricate tapestry of nature, fruit development is a delicate interplay of genetics, environment, and nutrition [1]. While the…...

In the intricate ballet of livestock nutrition, where every element plays a pivotal role in the animal's well-being and productivity,…...

What is Fine Chemicals? Fine chemicals are complex, single, pure chemical substances produced in limited quantities using specialized processes. They…...

Pidolic acid, also known as 5-oxoproline or L-pyroglutamic acid, is a naturally occurring amino acid derivative found in various tissues…...

What is Happiness Blueprint? In the pursuit of happiness, most of us look outward—toward relationships, success, or self-help routines. But…...

The importance of mineral chelates (nutrients) cannot be exaggerated. Factually, these nutrients are necessary for plant growth, development, and overall…...