Optimizing Vitamin C Stability and Permeability Using Liposomal Encapsulation
Liposomal vitamin C stability is no longer a formulation luxury; it has become a scientific necessity. It defines whether ascorbic acid performs as a therapeutic antioxidant or degrades into an unstable, ineffective byproduct before it even reaches its target.
Vitamin C (L-ascorbic acid) continues to dominate the antioxidant landscape in skincare, nutraceuticals, and pharmaceuticals. Yet, despite its unmatched biological relevance, its real-world performance is notoriously inconsistent. Why? Because Vitamin C is chemically fragile, biologically limited in absorption, and highly sensitive to environmental stressors.
This is where liposomal encapsulation changes the paradigm.
By combining biomimetic lipid bilayer architecture with nanoscale engineering, liposomal delivery systems fundamentally alter how Vitamin C behaves. Liposomal encapsulation ghastly enhances liposomal vitamin C stability, boost its permeability, and enables controlled release.
In this article, we explore the molecular science behind stable vitamin C formulations, examine the physicochemical metrics of ascorbic acid encapsulation, and reveal how WBCIL has engineered delivery systems that redefine liposomal vitamin C stability for clinical and cosmetic excellence.
Key Takeaways
- Bypassing the Absorption Ceiling: Liposomal encapsulation allows Vitamin C to bypass saturable sodium-dependent transporters (SVCTs) through membrane fusion and endocytosis, leading to up to 4-fold higher bioavailability.
- The Power of <200 nm Particles: Reducing particle size to below 200 nm dramatically enhances dermal penetration and mucosal adhesion, transforming Vitamin C from a surface-level active into a deep-tissue therapeutic.
- Molecular Shielding: High encapsulation efficiency (>80%) and a robust zeta potential (>-30 mV) create a stable micro-environment that protects the fragile enediol group of Vitamin C from heat, light, and oxidation.

The Physicochemical Constraints of Conventional Vitamin C
Before understanding why liposomal systems represent the best delivery system for vitamin C, we must first confront the biochemical vulnerabilities of conventional formulations.
- Oxidative Vulnerability: At the molecular level, Vitamin C is structurally unstable. The enediol group of L-ascorbic acid readily donates electrons – a property that grants it its antioxidant power but simultaneously makes it highly prone to oxidation.
Exposure to oxygen, light, heat, pH fluctuations, and metal ions rapidly destabilises active ascorbic acid into dehydroascorbic acid (DHA)
This explains why conventional formulations struggle with shelf life. Even minute oxidative triggers can degrade up to 50% of free Vitamin C within weeks, severely limiting its clinical efficacy.
- The Absorption Ceiling: Oral and topical absorption of Vitamin C is regulated by sodium-dependent vitamin C transporters (SVCT1 and SVCT2) [1]. These transporters operate on saturable kinetics, meaning once they reach capacity, additional doses offer diminishing returns.
This phenomenon explains why megadoses fail to improve plasma levels and topical application often delivers negligible dermal penetration
In short, conventional delivery hits a biological wall.
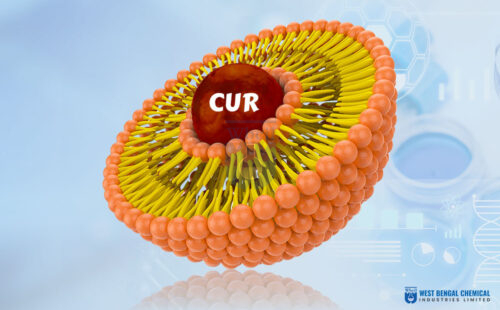
Engineering Stability: The Liposomal Micro-Environment
To overcome these constraints, liposomal encapsulation introduces a micro-environment that protects, transports, and releases Vitamin C in a controlled manner.
- Encapsulation Efficiency (EE) and core stability: Encapsulation Efficiency measures the fraction of Vitamin C successfully entrapped inside liposomal vesicles. High EE directly correlates with superior liposomal vitamin C stability [2].
- WBCIL Benchmark: While industry averages hover near 50%, WBCIL’s liposomal systems consistently achieve >80% encapsulation efficiency, ensuring nearly the entire dose is shielded from oxidative attack thus boosting liposomal vitamin C stability.
This remarkable achievement establishes our liposomal vitamin C as one of the most stable vitamin C formulations available to cosmeceutical and nutraceutical brands.
Through advanced ascorbic acid encapsulation, WBCIL effectively transforms Vitamin C into protected molecular cargo, dramatically extending shelf life and functional potency.
- Zeta Potential & Colloidal Stability: Zeta potential measures the surface charge of liposomes and reflects electrostatic repulsion between particles. If insufficient, particles aggregate, collapse, and the formulation destabilizes.
WBCIL Performance Metric:
- Zeta potential: > -30 mV
- Particle size: <200 nm
This combination ensures:
- Robust liposomal vitamin C stability
- Uniform particle distribution
- Prolonged shelf life
- Consistent release kinetics
Collectively, these parameters define the structural backbone of liposomal vitamin C stability.
Mechanisms of Enhanced Permeability
The real triumph of liposomal systems lies in their permeability, i.e., their ability to breach biological barriers.
Bypassing Saturable Active Transports (SVCTs)
Unlike conventional Vitamin C that relies on SVCT-mediated transport, liposomes exploit:
- Passive diffusion
- Endocytosis
- Membrane fusion
The phospholipid bilayer of liposomes integrates seamlessly with intestinal epithelial cells’/enterocytes’ cell membranes and skin lipid matrices. This bypass allows vitamin C encapsulation for enhanced permeability, resulting in dramatic improvements in absorption.
This is precisely why liposomal vitamin C vs normal vitamin C shows consistent superiority in both pharmacokinetic and clinical studies.
Mucosal Adhesion and Nano-Sized Penetration
Particle size governs cellular permeation.
| Parameter | Conventional Vitamin C | WBCIL Liposomal Vitamin C |
| Particle Size | ~824 nm | <200 nm |
| Skin Permeation | Low | High |
| Absorption Efficiency | Limited | Enhanced by 4 times |
This explains the superior skin penetration of vitamin C achieved using liposomal carriers.
By enabling deeper epidermal and dermal access, topical liposomal vitamin C achieves meaningful collagen synthesis, antioxidative defence, and photoprotection. Thus, delivering tangible clinical benefits [2].
Comparative Pharmacokinetics: Liposomal vs Conventional API
| Pharmacokinetic Parameter | Conventional Vitamin C | Liposomal Vitamin C |
| Bioavailability | Limited by saturable SVCT transport | Up to 4-fold higher bioavailability due to membrane fusion and endocytic uptake [3, 4] |
| Absorption Kinetics | Rapid absorption followed by steep decline | Gradual absorption with sustained plasma levels |
| Peak Plasma Profile | Sharp transient plasma spike | Broad concentration-time curve |
| Renal Clearance | Rapid renal excretion | Delayed clearance |
| Plasma Retention Time | Short-lived presence | 2.2x extended plasma persistence |
| Antioxidant Activity Duration | Brief and transient | Sustained antioxidant protection |
| Immune Modulation | Short-term immune stimulation | Prolonged immune-supportive effect |
| Dermal Exposure | Minimal systemic-to-skin delivery | Extended dermal exposure supporting skin repair |
| Release Pattern | Immediate release | Controlled release |
| Therapeutic Efficacy | Variable, short-lasting | Long-lasting, clinically meaningful efficacy |
Skin Science: Liposomal Vitamin C in Dermatology
Why Skin Needs Liposomal Delivery
The stratum corneum functions as a formidable permeability barrier. Free Vitamin C struggles to penetrate this lipid-rich environment, limiting biological impact.
Liposomal delivery resolves this challenge by incorporating natural membrane-mimicking phospholipids, enabling seamless lipid exchange. This mechanism dramatically enhances:
- Skin permeation of liposomal ascorbic acid [2]
- Optimizing topical vitamin C stability and absorption
Thus, liposomal vitamin C for skin penetration becomes not just beneficial but also essential.
Benefits of Liposomal Vitamin C for Skin
Clinically validated advantages include:
- Enhanced collagen synthesis [5]
- Accelerated photodamage repair [6]
- Reduction in oxidative stress
- Improved barrier integrity
- Long-lasting antioxidant defense
This makes liposomes the undisputed best delivery system for vitamin C in skincare
and why we see increased adoption of liposomal vitamin C among cosmeceutical brands.
Industrial Excellence at WBCIL: The R&D perspective
As a WHO-GMP and ISO-certified liposomal vitamin C manufacturer, WBCIL approaches liposomal formulation as molecular engineering rather than cosmetic blending.
To support this level of delivery-system engineering, WBCIL operates LipoEdge – its knowledge platform dedicated to advancing liposomal science. LipoEdge functions as a dynamic interface between laboratory research and industrial-scale formulation. For formulators and product developers, LipoEdge transforms theoretical liposomal science into actionable formulation intelligence- accelerating development timelines while ensuring performance predictability.
- Lecithin Purity & Bilayer Integrity: At WBCIL, we utilize non-GMO sunflower-derived phosphatidylcholine – a critical determinant of membrane fluidity, fusion efficiency, and oxidative protection.
This ensures:
- Structural stability
- Optimal particle formation
- Superior encapsulation efficiency
- Powdered Liposomal Stability: One of WBCIL’s most groundbreaking achievements lies in powdered liposomal technology.
By minimizing water activity (aᵥ), WBCIL:
- Prevents hydrolytic degradation
- Inhibits lipid oxidation
- Extends shelf life dramatically
This technological leap elevates stable vitamin C formulations into entirely new territory, unlocking logistics, storage, and formulation versatility.
For formulators, dermatologists, and nutraceutical developers, liposomal vitamin C stability defines product credibility. It determines whether a formulation delivers true biological performance or merely marketing claims. This positions WBCIL’s liposomal vitamin C at the forefront of formulation science.
Conclusion
The transition from conventional Vitamin C to liposomal encapsulation represents a foundational shift in pharmaceutical and cosmetic delivery science. By achieving unmatched liposomal vitamin C stability by optimizing zeta potential, minimizing particle size, and enabling controlled release, WBCIL has overcome every major biochemical limitation associated with L-ascorbic acid.
For formulators asking: How to stabilize vitamin C in formulations?
The answer is now clear: Through precision-engineered liposomal encapsulation.
For brands seeking: The most advanced vitamin C delivery platform?
The solution is unequivocal: WBCIL’s liposomal vitamin C.
As science continues to converge with skincare and nutrition, liposomal systems stand as the gold standard – delivering stability, permeability, and clinical excellence in one elegant molecular architecture.
👉 Partner with WBCIL- a trusted liposomal vitamin C manufacturer. Discover our liposomal vitamin C. Because when delivery respects biology, performance follows.
www.wbcil.com
1. Wang, Y., Mackenzie, B., Tsukaguchi, H., Weremowicz, S., Morton, C. C., & Hediger, M. A. (2000). Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochemical and biophysical research communications, 267(2), 488–494. https://doi.org/10.1006/bbrc.1999.1929
2. Llamedo, A., Rodríguez, P., de Passos, C., Freitas-Rodriguez, S., Coto, A. M., Soengas, R. G., & Alonso-Bartolomé, R. (2025). Liposomal formulation of a vitamin C derivative: a promising strategy to increase skin permeability. Journal of liposome research, 1–9. Advance online publication. https://doi.org/10.1080/08982104.2025.2466449
3. Łukawski, M., Dałek, P., Borowik, T., Foryś, A., Langner, M., Witkiewicz, W., & Przybyło, M. (2020). New oral liposomal vitamin C formulation: properties and bioavailability. Journal of liposome research, 30(3), 227–234. https://doi.org/10.1080/08982104.2019.1630642
4. Żmuda, P., Khaidakov, B., Krasowska, M., Czapska, K., Dobkowski, M., Guzowski, J., Kowalczyk, P., Lemke, K., Folwarski, M., Foryś, A., Domian, E., & Postuła, M. (2024). Bioavailability of Liposomal Vitamin C in Powder Form: A Randomized, Double-Blind, Cross-Over Trial. Applied Sciences, 14(17), 7718. https://doi.org/10.3390/app14177718
5. Maione-Silva, L., de Castro, E. G., Nascimento, T. L., Cintra, E. R., Moreira, L. C., Cintra, B. A. S., Valadares, M. C., & Lima, E. M. (2019). Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Scientific reports, 9(1), 522. https://doi.org/10.1038/s41598-018-36682-9
6. Lv, X., Wu, Z., & Qi, X. (2022). High skin permeation, deposition and whitening activity achieved by xanthan gum string vitamin c flexible liposomes for external application. International journal of pharmaceutics, 628, 122290. https://doi.org/10.1016/j.ijpharm.2022.122290
Yes. By encapsulating the ascorbic acid in a lipid shell, direct contact with the gastric mucosa is minimized, preventing the irritation associated with high-dose ingestion.
Scientific consensus points to particles under 200 nm. WBCIL targets a mean size of <200 nm to maximize mucosal and cellular uptake.
New research shows that while liposomes pick up a protein coat in the blood, the highly negative surface charge (Zeta potential) prevents interaction with serum proteins and improve cellular delivery.
High EE ensures minimal waste. Low EE means most of the Vitamin C is “free” in the solution and will degrade just as quickly as a standard supplement.
WBCIL’s powdered liposomal grade is specifically designed for thermal stability at room temperature, though liquid forms often require more controlled environments.
Absolutely. Liposomal encapsulation prevents Vitamin C from prematurely reacting with metal ions in the gut, thereby enhancing non-heme iron absorption.