The Industrial Powerhouse- Unveiling the Versatile Uses of Calcium Acetate
What is Calcium Acetate?
Calcium acetate, a seemingly simple compound with the formula Ca (CH3COO)2, might conjure up images of chalky supplements1. But beneath this unassuming exterior lies a surprisingly versatile industrial workhorse with applications far exceeding role of calcium acetate supplements in bone health. Determining the optimal calcium acetate dosage depends on the specific application, whether Calcium Acetate in food preservation or use of calcium acetate tablet in managing phosphorus levels in patients with kidney disease.2 3
While calcium acetate supplements effectively bind excess phosphate, nurses must be aware of potential side effects and closely monitor serum calcium levels for safe and effective use with their patients considering intact “calcium acetate nursing considerations.”
Properties of Calcium Acetate
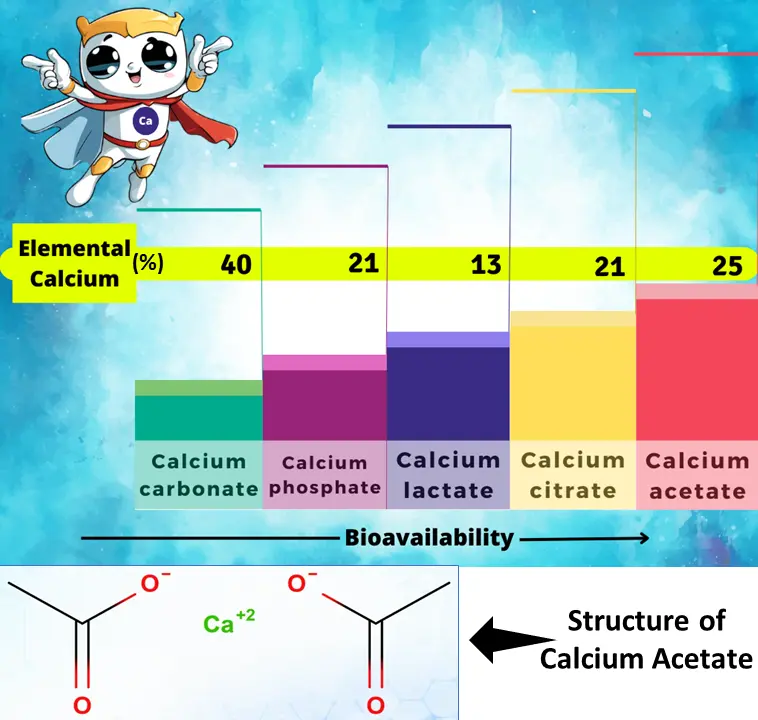
Calcium acetate IUPAC name is calcium diacetate. It exists as a salt, meaning it forms from the attraction between positively charged calcium ions (Ca²⁺) and negatively charged acetate ions (CH3COO⁻). Each calcium ion interacts with two acetate ions to balance the charges4. Calcium acetate molecular formula for calcium acetate monohydrate is Ca (CH3COO) ₂·H₂O 5. Calcium acetate preparation follows a reaction between calcium and acetic acid Calcium acetate chemical formula is Ca (CH3COO)2 with calcium acetate molar mass 158.138 g/mol. Each acetate ion has a carbon atom at its core, bonded to two hydrogen atoms and a carbonyl group (C=O). Calcium acetate CAS no. is 62-54-4 6. Calcium acetate density is 1.509 g/cm³. It remains solid until reaching Calcium acetate melting point of 160°C.
Calcium Acetate solubility is highest among these options.6 This means calcium acetate in water dissolves very easily, making it readily available for absorption by the body.

The Multifaceted Role of Calcium Acetate Across Industries
1️⃣ From Food Additive to Mold Foe: A Culinary Ally:
Calcium acetate for medication is not the only use. In the culinary world, it takes centre stage as a food additive (E263) known for its multiple functionalities. It acts as a firming agent, enhancing the texture of jams, jellies, and candies7. Calcium acetate acts as a stabilizer, preventing discoloration and ensuring a consistent product. It also functions as a buffer, maintaining the desired acidity in foods like salad dressings and pickles. However, beyond aesthetics and preservation, it plays a crucial role in preventing the growth of mold and spoilage in bread.
2️⃣ A Stabilizer for Resins and Buffer for Beverages:
In the world of industrial chemistry, it serves as a vital stabilizer in resins, used for adhesives to paints. Calcium acetate acts as a savior, preventing this degradation and ensuring the long-term integrity of these crucial materials. Some beverages, particularly carbonated drinks, can become acidic over time that affects taste and can also corrode containers. Calcium acetate acts as a buffer, maintaining a consistent pH level and protecting both the beverage and its packaging.
3️⃣ Soothing Relief:
When combined in water, calcium acetate and aluminum sulfate create a chemical reaction. This results in a calming solution that relieves itching and inflammation from mild skin issues like poison ivy. The mixture works by constricting tissues, reducing swelling, and promoting healing.
4️⃣ Lubricant Manufacturing:
Imagine fat or oil and an alkali having a reaction, breaking down and forming soap molecules. Calcium acetate steps in, influencing the size and shape of these molecules, which determines the final thickness of the grease. it prevents the formation of rust and other corrosive elements that can damage machinery. It can even act as a shield in some lubricants, reducing wear on metal surfaces.
5️⃣ Plastics Industry:
Imagine turning heavy PVC into a lightweight sponge! Calcium acetate acts like a magic trick, breaking down when heated to release tiny gas bubbles. These bubbles get trapped inside the PVC, creating a foamy texture. The process is precise, with calcium acetate decomposing at a controlled rate for just the right amount of air. This results in a fine, even distribution of bubbles, making the PVC foam strong yet flexible.
6️⃣ Printing Ink Industry:
Calcium acetate acts like a secret weapon for ink makers! This can make the final ink stick better, last longer, and even resist fading from sunlight. But calcium acetate’s uses don’t stop there! It can also help fine-tune the acidity of the mixture, which is crucial for the ink’s quality. In some cases, it might even help remove unwanted impurities.
7️⃣ Water Treatment
Clean Tiny troublemakers in your water? Calcium acetate to the rescue! This clever chemical acts like a matchmaker, grabbing hold of small impurities and clumping them together. Imagine it floating around with sticky arms, connecting clay particles, bacteria, and other nasties. These bigger clumps, called flocs, become heavy and sink to the bottom, making them easy to scoop out.
8️⃣ Concrete Setting
Normally, concrete takes time to harden fully. But calcium acetate acts like a booster, speeding up this process. This can be crucial in construction projects where time is tight. Calcium magnesium acetate, a chloride-free alternative for de-icing, is gaining popularity due to its lower corrosiveness on concrete and metal infrastructures.
9️⃣ Textile Industry
Calcium acetate plays a surprising role in dyeing! It acts like a double-sided tape, helping dyes stick better to fabrics. This “mordant” makes colors richer and longer-lasting, ensuring your favorite shirt stays vibrant.
🔟 Agriculture
Calcium acetate plays a surprising role on some farms! It can act as a double agent: delivering calcium, a crucial nutrient for plant growth, and helping fight against blossom end rot, a nasty condition that harms fruits and vegetables. This calcium boost strengthens cell walls and helps plants take up other nutrients better. It’s like giving them a healthy dose of vitamins and a shield against weakness!
1️⃣1️⃣ A Historical Hero
Before the development of the cumene process in the mid-20th century, calcium acetate was a key player in acetone production. Acetone, a crucial solvent used in various industries, was traditionally obtained by heating calcium acetate.

Conclusion
Calcium acetate’s journey from a forgotten chemistry experiment to a versatile industrial player is a testament to the power of scientific exploration. Its diverse applications, from enhancing our food to protecting our machinery, showcase the remarkable range of this seemingly simple compound. While generally well-tolerated, some users may experience mild calcium acetate side effects like nausea or constipation. As research continues, we can expect even more unexpected uses for calcium acetate to emerge in the years to come.
-
Mai ML, Emmett M, Sheikh MS, Santa Ana CA, Schiller L, Fordtran JS. Calcium acetate, an effective phosphorus binder in patients with renal failure. Kidney Int. 1989 Oct;36(4):690-5. doi: 10.1038/ki.1989.247. PMID: 2811066. https://pubmed.ncbi.nlm.nih.gov/2811066/
-
Wikipedia contributors. Calcium acetate. Wikipedia, The Free Encyclopedia. March 3, 2024, 23:45 UTC. Available at: https://en.wikipedia.org/w/index.php?title=Calcium_acetate&oldid=1211700415. Accessed May 13, 2024.
-
National Center for Biotechnology Information. PubChem Compound Summary for CID 6116, Calcium Acetate. https://pubchem.ncbi.nlm.nih.gov/compound/Calcium-Acetate
-
https://www.webmd.com/drugs/2/drug-178358/calcium-acetate-oral/details
Calcium acetate is used in food preservation, resin stabilization, beverage buffering, skin irritation relief, lubricant manufacturing, the plastics industry, printing inks, water treatment, concrete setting, the textile industry, and agriculture.
As a food additive (E263), calcium acetate acts as a firming agent, stabilizer, buffer, and mold inhibitor in products like jams, jellies, candies, salad dressings, pickles, and bread.
Calcium acetate tablets are used to bind excess phosphate in the body, helping manage phosphorus levels in patients with kidney disease.
Common side effects include nausea and constipation. Nurses must monitor serum calcium levels to ensure safe and effective use
Calcium acetate influences the size and shape of soap molecules during the reaction of fat or oil with an alkali, determining the final thickness of the grease and preventing corrosion and wear on metal surfaces.
In the printing ink industry, calcium acetate improves ink adhesion, longevity, resistance to fading, and fine-tunes the acidity of the mixture. It can also help remove impurities.
Calcium acetate acts as a coagulant, grabbing hold of impurities and clumping them together into larger flocs that sink to the bottom, making them easier to remove.
Before the cumene process was developed, acetone was produced by heating calcium acetate, highlighting its importance in early industrial chemistry.
In agriculture, calcium acetate delivers calcium to plants, strengthening cell walls and preventing conditions like blossom end rot, while also aiding nutrient uptake.
