-
Product Name:
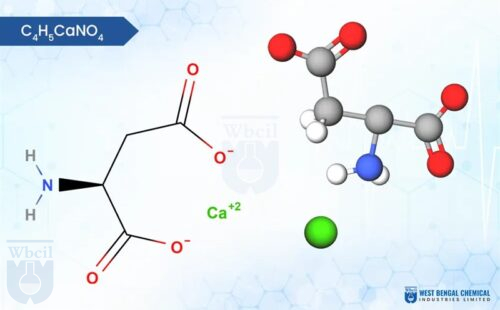
Calcium Acetate
-
Molecular Formula:
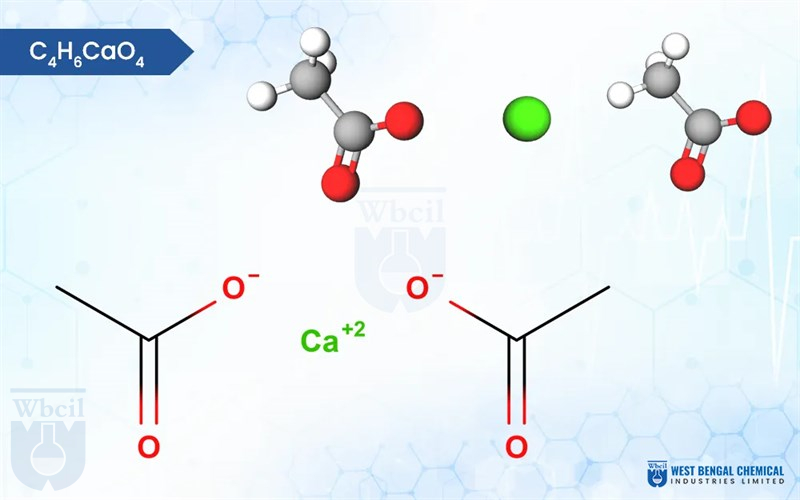
C4H6CaO4
-
Molecular Weight:
158.17 g/mol
-
CAS No.:
62-54-4
-
HSN Code:
29152910
-
CID Code:
6116
-
Shelf Life:
3 years - 20°C powder
-
ATC Code (WHO)
V03AE07
-
DrugBank ID
DB00258
-
ChemSpider ID
5890
-
UNII No.
Y882YXF34X
- USP
- IUPAC Names
- Synonyms
- MSDS
- Specification
USP of Calcium Acetate
- Calcium acetate binds excess phosphate in kidney failure, phosphate binder in CKD patients.
- Used as calcium supplementation.
- Regulates acidity and firm’s food textures, a win for taste and shelf-life.
- Inhibits metal corrosion, safeguarding infrastructure and extending lifespans.
- Plays important role as a cation in manufacturing statins.
- Catalyses esterification and aids lubricant production, a scientific engine for industry.
IUPAC Names of Calcium Acetate
calcium;diacetate
Synonyms of Calcium Acetate
- Calcium acetate
- 62-54-4
- Calcium diacetate
- Acetic acid, calcium salt
- Lime acetate
- Lime pyrolignite
- Acetate of lime
- calcium ethanoate
- calcium;diacetate
- Brown acetate
- acetic acid calcium salt
- calcium acetate salt
- Calcium di(acetate)
- calciumacetate
MSDS of Calcium Acetate
Download MSDS PDF- MSDS Name: Calcium Acetate
- Product Code: CAEIH99 (IN HOUSE)
- Relevant identified uses of the substance or mixture and uses advised against: Laboratory chemicals, Industrial & for
professional use only. - CAS No.: 62-54-4
- General advice Consult a physician: Show this safety data sheet to the doctor in attendance.
- If inhaled: If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
- In case of skin contact: Wash off with soap and plenty of water. Consult a physician.
- In case of eye contact: Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
- If swallowed: Never give anything by mouth to an unconscious person. Rinse mouth with water.
- Most important symptoms and effects, both acute and delayed:
The most important known symptoms and effects are described in the labeling and/or in section 11 - Indication of any immediate medical attention and special treatment needed: no data available
- Suitable extinguishing media: Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
- Special hazards arising from the substance or mixture: Carbon oxides, Calcium oxide
- Advice for firefighters: Wear self-contained breathing apparatus for firefighting if necessary.
- Further information: No data available
- Control parameters: Components with workplace control parameters
- Appropriate engineering controls: Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
- Eye/face protection: Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
- Skin protection: Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove’s outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
- Body Protection: impervious clothing, the type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace.
- Respiratory protection: For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator. For higher level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU).
- Control of environmental exposure: Do not let product enter drains.
- Appearance Form: Solid, powder in nature.
- Color: White
- Solubility: Soluble in water
- Other safety information: No data available
- https://whmis.org/sds/
- https://www.osha.gov/sites/default/files/publications/OSHA3514.pdf
- https://reachonline.eu/reach/en/annex-ii.html
- https://www.cdc.gov/niosh/npg/
- https://echa.europa.eu/registration-dossier/-/registered-dossier/13608
- https://en.wikipedia.org/wiki/Calcium_acetate
- https://pubchem.ncbi.nlm.nih.gov/compound/Calcium-Acetate
- https://webapps.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=1092&p_version=2
- https://comptox.epa.gov/dashboard/chemical/details/DTXSID0020234
Description of Calcium Acetate
Calcium acetate, with the chemical formula Ca(CH3COO)2, is a white, crystalline salt or granules formed by the reaction of acetic acid (CH3COOH) with calcium hydroxide (Ca(OH)2). It possesses a faint vinegar-like odor due to the presence of acetic acid. Calcium acetate is hygroscopic, and exhibits a slightly chalky texture. Calcium acetate, a powerful phosphate binder specifically formulated for CKD patients maintain optimal kidney health.
It is a calcium salt of acetic acid. It is soluble in water and slightly soluble in ethanol. Calcium acetate exists in both anhydrous and monohydrate forms. The monohydrate form, Ca(CH3COO)2·H2O, is the most commonly encountered version. In its anhydrous form, calcium acetate has a hygroscopic nature, readily absorbing moisture from the surrounding environment. Moreover, calcium acetate excels in metal chelation due to its propensity for Lewis acid-base interactions.
This exceptional chelating property positions it as a premier sequestrant in various applications. The safety profile and unique properties of our calcium acetate make it a valuable food additive. It finds uses in acidity regulation, flavour enhancement, and more. As a leading manufacturer from 1962, we are committed to the highest quality standards (CGMP and ISO certified). Our rigorous quality control processes ensure you receive pure and potent calcium acetate you can trust.
What is calcium acetate chemical formula?
Here is the calcium acetate chemical formula: C4H6O4.Ca or C4H6CaO4
Calcium Acetate uses
Calcium acetate, with the chemical formula Ca(C2H3O2)2, is a calcium salt widely used for both medical and industrial purposes. This compound has a variety of applications, particularly in medicine, where it is frequently prescribed for people with kidney conditions. Let’s explore the key benefits and common uses of calcium acetate.
- Phosphate Binder: Treats hyperphosphatemia by binding dietary phosphate.
- Parathyroid Hormone (PTH) Regulation: Reduces phosphate levels, normalizing PTH production.
- Soft Tissue Calcification Prevention: Prevents abnormal calcium-phosphorus deposition in tissues.
- Statin Drug Counterion: Used to form calcium salts in certain statin medications.
- Firming Agent: Stabilizes textures in jams, jellies, and candies.
- Stabilizer: Prevents discoloration and ensures consistency.
- Buffer: Maintains acidity in dressings and pickles.
- Mold Inhibitor: Prevents mold growth and spoilage in bread and baked goods.
- Calcium Supplementation: Strengthens cell walls, enhances disease resistance, and improves nutrient uptake (e.g., phosphorus, potassium).
- Foliar Spray and Fertilizer Additive: Provides sustained calcium release for optimal plant health.
- Pest Control: Attracts pests like yellow jackets for effective control.
- Soil pH Adjustment: Raises soil pH, improving crop suitability.
- Crop-Specific Benefits:
- Prevents disorders (e.g., tip burn in leafy greens, blossom end rot in tomatoes, bitter pit in apples).
- Enhances fruit quality, shelf life, and storage (e.g., grapes, plums, figs).
- Improves root development and color in crops like beets.
Enhances grease consistency, water resistance, and lubrication properties in:
- Automotive Components: Wheel bearings and chassis systems.
- Heavy Machinery: Gears and bearings in construction and mining equipment.
- Industrial Applications: General machinery requiring durable lubrication.
Improves the durability and stability of resins and adhesives.
Acts as a chelating agent to remove heavy metals from wastewater, supporting environmental safety.
Enhances fabric quality and durability during dyeing and finishing processes.
Protects metal surfaces from corrosion in industrial environments
Benefits of calcium acetate formula and its uses
- Food Preservative (E263): Acts as a preservative, extending the shelf life of foods by inhibiting bacterial growth.
- Acidity Regulator: Helps balance pH levels in food products, maintaining their quality and taste.
- Stabilizer in Food Processing: Ensures consistency and texture in processed foods, preventing separation or degradation during storage.
- Soap and Lubricant Manufacturing: Used as a binding agent, improving the texture and performance of soaps and lubricants.
- Textile Industry: Employed in dyeing processes to enhance the absorption of dyes in fabrics.
- Concrete Production: Serves as a component in some concrete mixes to control the setting time and improve strength.
Calcium acetate is utilized in the food industry as a preservative and stabilizer. In pharmaceuticals, it serves as an excipient. It’s also used in construction materials, water treatment, and as a laboratory reagent.
Yes, our calcium acetate meets the quality standards required for both food additives and pharmaceutical excipients, ensuring safety and efficacy for these applications.
The minimum order quantity can vary based on your requirements. Please contact our sales team for more information and to discuss your specific needs.
Orders can be placed directly through our website, via email, or by contacting our customer service team. We’re here to ensure a smooth ordering process.
Our calcium acetate is of high purity, typically ≥99.0% (calculated on dried material). It is available in a hygroscopic, crystalline solid or powder form.
Calcium acetate should be stored in a cool, dry place, away from incompatible substances. It is typically packaged in sealed containers to maintain its quality and extend shelf life.
Absolutely! We provide comprehensive technical support and can assist with formulation challenges, application development, and any other technical inquiries you may have.