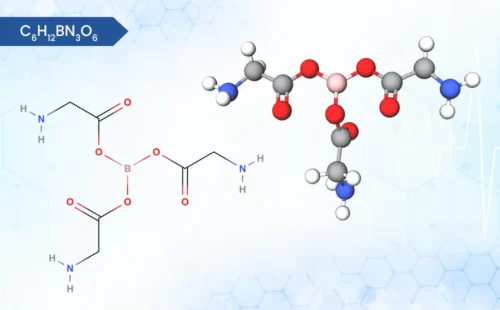
Zinc Bisglycinate
-
Product Name:
Zinc Bisglycinate
-
Molecular Formula:
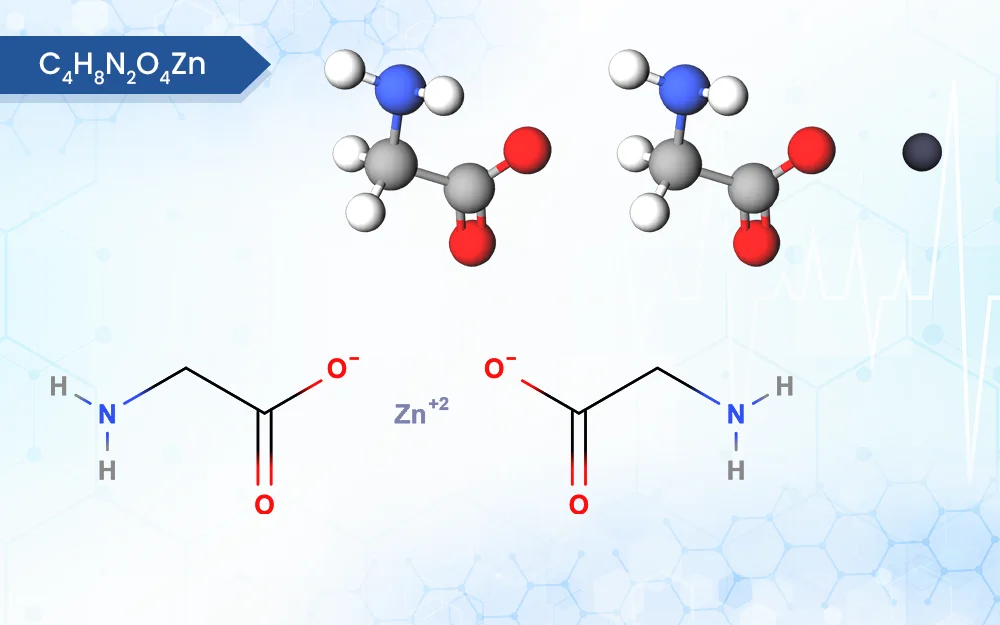
C4H8N2O4Zn
-
Molecular Weight:
213.51 g/mol
-
CAS No.:
14281-83-5
-
HSN Code:
29224990
-
CID Code:
151910
-
Shelf Life:
3 years - 20°C powder
-
DrugBank ID
DB14493
-
ChemSpider ID
133891
-
UNII No.
681VJX72FE
- USP
- IUPAC Names
- Synonyms
- MSDS
USP of Zinc Bisglycinate
- This form of zinc is often used as a dietary supplement and is known for its high bioavailability.
- Zinc bisglycinate is absorbed in the small intestine. The presence of glycine in the compound aids in the transport of zinc through the intestinal wall, leading to higher absorption rates compared to other forms of zinc.
IUPAC Names of Zinc Bisglycinate
zinc;2-aminoacetate
Synonyms of Zinc Bisglycinate
- Zinc glycinate
- zinc glycine chelate
- zinc;2-aminoacetate
- Glycine Zinc Salt
- zinc bisglycinate
- Zinc(II) glycinate
- Zinc Chelazome
- Bis(glycinato-N,O)zinc
- Zinc(II) 2-aminoacetate
- zinc(2+) bis(2-aminoacetate)
- (T-4)-Bis(glycinato-N,O)zinc
- zinc 2-aminoacetate
- Zincglycinate
- UNII-681VJX72FE
- Zinc Bis-Glycinate
- zinc 2-azanylethanoate
- bis(glycinato)zinc
- Glycine Zinc Chelate
- Glycine Zinc Salt (2:1)
- Zinc Bis (Aminoacetate)
- Glycine Zinc Salt Monohydrate [for Protein Research]
- Zinc Amino Acid Chelate
- Zinc, bis(glycinato-kappaN,kappaO)-, (T-4)-
MSDS of Zinc Bisglycinate
Download MSDS PDFFire-Fighting Measures
- Flammability of the Product: May be combustible at high temperature.
- Auto-Ignition Temperature: Not available.
- Flash Points: Not available.
- Flammable Limits: These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2…). Some metallic oxides.
- Products of Combustion: Slightly flammable to flammable in presence of heat. Non-flammable in presence of shocks.
- Fire Hazards in Presence of Various Substances: Slightly explosive in presence of open flames and sparks. Non-explosive in presence of shocks.
- Fire Fighting Media and Instructions:
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. Do not use water jet. - Special Remarks on Fire Hazards: Fire is possible at elevated temperatures.
- Special Remarks on Explosion Hazards: Fine dust dispersed in air in sufficient concentrations, and in the presences of an ignition source is a potential dust explosion hazard.
Exposure Controls / Personal Protection
- Engineering Controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit.
- Personal Protection: Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves.
- Personal Protection in Case of a Large Spill: Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self-contained breathing apparatus should be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product.
Physical and Chemical Properties
- Form: Free flowing powder, hygroscopic in nature.
- Color: White to off
- pH: 5.0 – 7.5
Stability and Reactivity
- Stability: The product is stable.
- Instability Temperature: Not available.
- Conditions of Instability: Excess heat, excess dust generation, incompatible materials.
- Incompatibility with various substances: Reactive with acids.
- Corrosivity: Not available.
- Special Remarks on reactivity: Incompatible with strong mineral acids, and strong organic acids.
- Special Remarks on Corrosivity: Not available.
- Polymerization: Will not occur
If you are interested on Zinc Bisglycinate, then
Description of Zinc Bisglycinate
Zinc Bisglycinate is a chelated form of zinc, it consists of a zinc ion bonded to two glycine molecules.
Application of Zinc Bisglycinate
- Helps in maintaining overall health, immune function, and various enzymatic processes within the body.
- Treatment for Irritable bowel syndrome (IBS) or other digestive disorders
If you are interested on Zinc Bisglycinate, then
Related Products
Related Blogs of Zinc Bisglycinate

Zinc Acetate Commonly occurring as the dihydrate Zn(CH3CO2)2•2H2O, zinc acetate is a salt that has the chemical formula of Zn(CH3CO2)2.…...

What is Zinc Citrate? Produced by complete neutralization of psychiatric acid with the highly pure zinc source, subsequent precipitation, and…...

Iron Glycine Chelated Minerals and its usage in the agriculture industry Iron Glycine chelated mineral is generally used in the…...