-
Product Name:
Ferrous Glycine Sulphate
-
Molecular Formula:
C4H10FeN2O8S
-
Molecular Weight:
302.04 g/mol
-
CAS No.:
17169-60-7
-
HSN Code:
28331990
-
CID Code:
167159
-
Shelf Life:
3 years - 20°C powder
-
ATC Code (WHO)
B03AA01
-
ChemSpider ID
26388
-
UNII No.
56PS9HY5XI
- USP
- IUPAC Names
- Synonyms
- MSDS
USP of Ferrous Glycine Sulphate
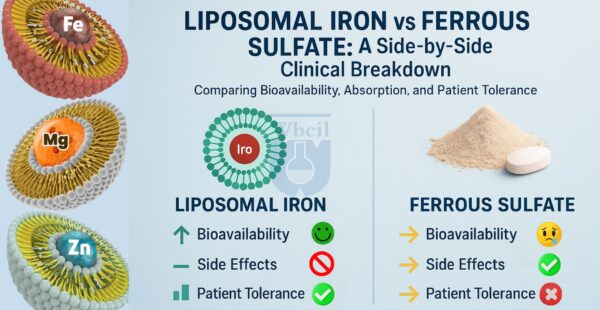
- It has significantly higher iron absorption due to its chelated form.
- It causes fewer gastrointestinal side effects than other iron supplements.
- Patients are more likely to take it consistently due to better tolerability.
- The chelation protects iron in acidic stomach conditions, ensuring efficient delivery.
- It utilizes a unique absorption pathway via amino acid transporters.
- Its stability makes it excellent for fortifying both human food and animal feed.
- Efficient absorption means less free iron in the gut, reducing oxidative damage.
IUPAC Names of Ferrous Glycine Sulphate
Iron(2+) sulphate, compound with glycine (1:2)
Synonyms of Ferrous Glycine Sulphate
- Ferrous glycine sulfate
- FERROUS GLYCINE SULFATE [WHO-DD]
- FERROUS GLYCINE SULPHATE
- Iron(ii) glycine sulfate
- IRON, BIS(GLYCINATO)-, SULFATE (1:1)
- UNII-56PS9HY5XI
- 14729-84-1
- Iron(2+) sulphate, compound with glycine (1:2)
- Ferrous sulfate glycine
MSDS of Ferrous Glycine Sulphate
Download MSDS PDF- MSDS Name: Ferrous Glycine Sulphate
- Product Code: FGSNL22
- Relevant identified uses of the substance or mixture and uses advised against: Industrial uses
- Inhalation: Move exposed person to fresh air. If not breathing, seek immediate medical attention. If breathing is irregular or if respiratory arrest occurs, provide artificial respiration or oxygen by trained personnel and seek medical attention.
- Ingestion: Do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Seek medical attention.
- Skin contact: Remove contaminated clothing and shoes and immediately flush skin with plenty of water for at least 15 minutes. Wash clothing before reuse.
- Eye contact: Check for and remove any contact lenses. Immediately flush eyes with plenty of water for at least 15 minutes.
- Flammability of the Product: Non-flammable.
- Fire extinguishing media: Use fire-extinguishing media appropriate to the surrounding fire.
- Products of Combustion: Carbon oxides, Nitrogen oxides, Sulfur oxides, Iron Oxides, etc.
- Fire Extinguishing Media: Use water spray, alcohol-resistant foam, dry chemicals, or carbon dioxide. Use means suitable for extinguishing surrounding fire. Avoid solid water jet as it may scatter the fire.
- Hazardous combustion products: Decomposition products may include oxides of carbon and iron.
- Special information: In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full-face piece operated in the pressure demand or other positive pressure mode.
- Engineering controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume, or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit.
- Personal protection: Safety glasses, Lab Coat, Dust respirator. Be sure to use an approved / certified respirator or equivalent, Gloves.
- Personal protection in case of a large spill: Splash goggles, Full suit, Dust respirator, Boots, Gloves. A self-contained breathing apparatus should be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product.
- Exposure limits: Consult local authorities for acceptable exposure limits.
- Skin Protection: Wear fire/flame resistant and impervious clothing. Handle with gloves. Gloves must be inspected prior to use. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
- Eye Protection: Use chemical safety goggles and/or full-face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area.
- Exposure limits: Consult local authorities for acceptable exposure limits.
- Appearance: Light yellow to brown
- Odor: Odourless
- pH: 3-5
- Initial boiling point and boiling range: 240.9°C at 760mmHg.
- Flash point: 99.5°C
- Vapor pressure: 0.0123mmHg at 25°C
- Solubility: It is soluble in water.
Description of Ferrous Glycine Sulphate
Ferrous glycine sulphate is a complex organic compound, widely utilized as an iron supplement due to its enhanced bioavailability. Its overall chemical structure involves a ferrous iron (Fe2+) ion complexed with glycine molecules and a sulfate (SO42−) ion.
The most commonly accepted molecular formula for ferrous glycine sulphate is C4H10FeN2O8S, representing a complex where two glycine molecules are associated with one ferrous sulfate unit. It commonly appears as a crystalline powder, with reported colors ranging from yellowish-green to brownish-green, greyish-brown, buff, or even light yellow to off-white. It generally presents with a mild, specific, and sometimes sweetish taste, and is largely considered odorless.
A significant technical characteristic is its hygroscopicity, meaning it readily absorbs moisture from the atmosphere. This property necessitates storage in well-closed, dry containers protected from light to maintain its stability and efficacy. In aqueous solutions, it typically forms a clear solution and exhibits an acidic pH, often in the range of 3.0 to 5.0 for a 5% solution.
- Primary use as an oral iron supplement for preventing and treating iron deficiency anemia.
- Prescribed to pregnant women for increased iron requirements.
- Used to replenish iron stores after surgery.
- Aids iron levels during recovery from illness.
- Primarily as a nutrient supplement and fortificant, improving nutritional profiles without altering taste or appearance.
- Used to enrich foods like milk, juices, and baked goods with iron.
- Extensively used in animal feed as an iron supplement for:
- Poultry: Improves growth, feed intake, and red blood cell parameters.
- Weaned Piglets: Addresses iron deficiency, prevents anemia, and increases weight gain.
- Other Livestock: Prevents and corrects iron deficiency, supporting healthy growth.
- Valuable reagent in chemical synthesis and research.
- Uses where a stable and bioavailable ferrous iron source is needed.